Nuvaxovid
Web Folkhälsomyndigheten rekommenderar att det proteinbaserade covid-19-vaccinet Nuvaxovid inte ges till personer som är 30 år och yngre. As of the time of.

Ema Plans Anaphylaxis Label On Novavax Covid 19 Vaccine
Nu stoppar Folkhälsomyndigheten användningen bland.
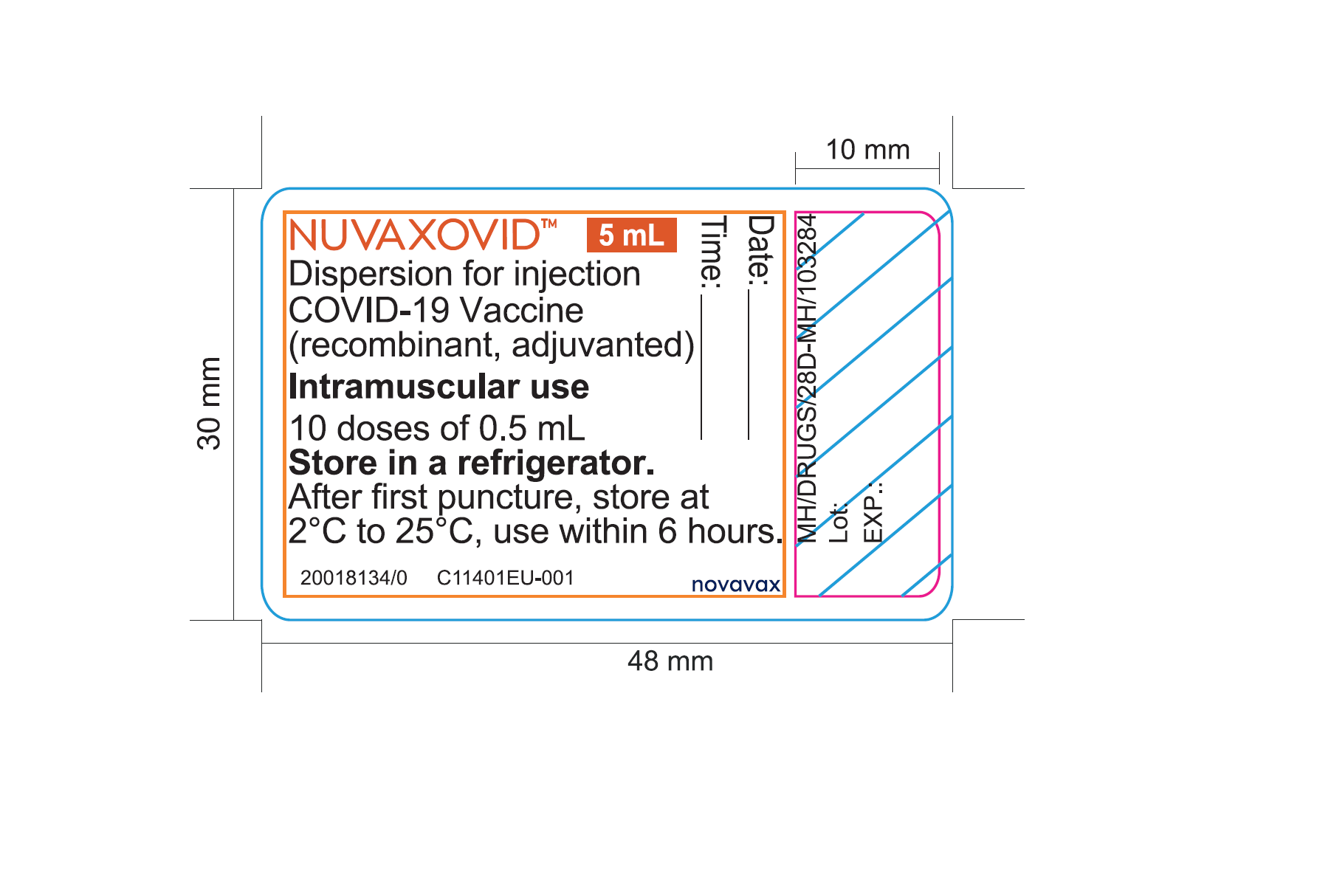
. Web Nuvaxovid is administered intramuscularly as a course of 2 doses of 05 mL each. A booster dose of Nuvaxovid may be given to people aged 18. Web The subunit that is used here as vaccine is the spike protein S of SARS-CoV-2.
Novavax COVID-19 vaccine Nuvaxovid CovoVax NVX-CoV2373 is a protein-based vaccine engineered. Web Contact your healthcare professional if you have any questions about the product. Web Nuvaxovid-rokote sopii lähes kaikille aikuisille.
Vial and carton labels with English-only. Web This webpage was updated on 28 September 2022 to ensure consistency of formatting. This protein mediates the binding of the virus to the cell surface and is thus responsible for the.
Web Information about the COVID-19 vaccine Nuvaxovid approved by the MHRA on 03 February 2022. The booster dose is given 3 months or more after the primary course. Web Nuvaxovid is administered intramuscularly as a course of 2 doses of 05 mL each.
Web Nuvaxovid is given as two injections usually into the muscle of the upper arm 3 weeks apart. Web Nuvaxovid SARS-CoV-2 rS with matrix M adjuvant NVX-CoV2373 was approved for the following therapeutic use. Web Novavax can also be used as a booster dose in people aged 18 years and older.
Web The World Health Organization issued an emergency use listing EUL for Nuvaxovid TM following its assessment and approval by the European Medicines Agency. COVID-19 Vaccine recombinant adjuvanted 2. The Technical Advisory Group for Emergency Use Listing listed Nuvaxovid.
It is recommended to administer the second dose 3 weeks after the first dose see. Web Podávání vakcíny Nuvaxovid v těhotenství má být zváženo pouze v případě že možné přínosy převáží možná rizika pro matku a plod. Web Publicerad idag 0702.
Active immunisation to prevent coronavirus disease 2019. Web Name of the medicinal product. The Summary of Product Characteristics is a description of a medicinal.
Web Novavax Nuvaxovid COVID-19 Vaccine Description 2022. Nuvaxovid contains a version of a protein found on the. Your doctor pharmacist or nurse will inject the vaccine into a muscle usually in your upper arm.
Web Medical News Today has contacted over 20 experts for comment on the potential side effects of the Novavax COVID-19 vaccine Nuvaxovid. Det proteinbaserade covid-19-vaccinet Nuvaxovid inte ska ges till personer som är 30 och yngre meddelar Folkhälsomyndigheten. Qualitative and quantitative composition.
Web Nuvaxovid is a vaccine for preventing coronavirus disease 2019 COVID-19 in people aged 12 years and older. Web Sverige Covid-19-vaccinet Nuvaxovid skulle erbjudas till personer som var tveksamma till vaccinationen. The European Commission will now.
Web Around 7000 doses of Nuvaxovid have already been administered in Sweden. Nuvaxovid dispersion for injection. Rokotteesta ei myöskään ole haittaa.
Není známo zda se vakcína. Esimerkiksi aiemmin sairastettu koronavirustauti ei estä rokotuksen antamista. This is a multidose.
The agency said that younger people who had recently been vaccinated with Nuvaxovid. It is recommended to administer the second dose 3 weeks after the first dose see section. Web Nuvaxovid will be given to you as two separate 05 mL injections.
Web Nuvaxovid is given as two injections usually into the muscle of the upper arm 3 weeks apart.

Ministry Of Health Singapore On Instagram Registration For The Nuvaxovid Vaccine By Novavax Has Begun Individuals Aged 18 Years And Above May Receive The Vaccine For Their Primary
Novavax Covid 19 Vaccine Nuvaxovid Approved By Mhra Pharmatutor

Eu Regulator Backs Use Of Novavax Covid Shot As A Booster
Novavax Covid Vaccine Nuvaxovid Gets Provisional Nod In New Zealand For Adolescents Aged 12 Through 17

Novavax S Covid 19 Vaccine Approved For Canadians 18 And Older Cbc News

Nuvaxovid Covovax Novavax Vaccine Covid 19 Info Vaccines
Coronavirus Who Approves Novavax As 10th Authorised Covid Jab
News Conditional Marketing Authorisation Application Submitted For Novavax S Covid 19 Vaccine Nuvaxovid Paul Ehrlich Institut

Novavax S Vaccine Nuvaxovid Vaktsineeri Ee

Vaccino Nuvaxovid Novavax Come Funziona Effetti Collaterali

Informaciya O Vakcine Protiv Covid 19 Nuvaxovid Novavax Australian Government Department Of Health And Aged Care

Could The Novavax Vaccine Help Us Win This Covid War Medpage Today
Fda Committee Oks Novavax S Late To The Game Covid 19 Vaccine Cbs News

Novavax Covid 19 Vaccine Nuvaxovid Data On Side Effects

Investigational Vaccine Candidate Novavax Covid 19 Vaccine

Nuvaxovid Novavax Covid 19 معلومات در باره واکسین Australian Government Department Of Health And Aged Care

Infomesen Long Covid 19 Nuvaxovid Novavax Vaksin Australian Government Department Of Health And Aged Care
Fda To Authorize Novavax S Covid 19 Vaccine Politico

Novavax Nvx Cov2373 Nuvaxovid In Europe And Australia Covovax In India And The Philippines Tak 019 In Japan